orbitals s p d f|o que são orbitais : Clark The simplest atomic orbitals are those that are calculated for systems with a single electron, such as the hydrogen atom. An atom of . Tingnan ang higit pa Rajacukong88: Situs Judi Slot Online Terbaik Dan Terpercaya 2023. Link rajacukong merupakan situs judi slot online terpercaya indonesia dengan kemenangan predikat rating tertinggi bisa di lihat dari RTP live cukong slot. Volatilitas dengan panduan rtp live 88 cukong raja slot online telah menjadi panduan para pemain kami.
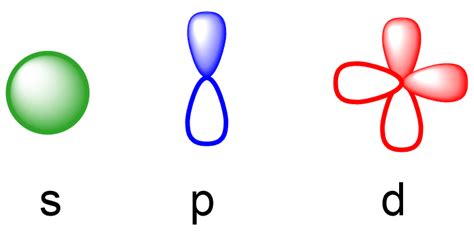
orbitals s p d f,The simple names s orbital, p orbital, d orbital, and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2, and 3 respectively. These names, together with the value of n, are used to describe the electron configurations of atoms. Tingnan ang higit paIn quantum mechanics, an atomic orbital is a function describing the location and wave-like behavior of an electron in an atom. This function describes the electron's Tingnan ang higit pa

The term "orbital" was coined by Robert S. Mulliken in 1932 as short for one-electron orbital wave function. Niels Bohr Tingnan ang higit paThe simplest atomic orbitals are those that are calculated for systems with a single electron, such as the hydrogen atom. An atom of . Tingnan ang higit pa
Simple pictures showing orbital shapes are intended to describe the angular forms of regions in space where the electrons occupying . Tingnan ang higit pao que são orbitaisWith the development of quantum mechanics and experimental findings (such as the two slit diffraction of electrons), it was found that the electrons orbiting a nucleus . Tingnan ang higit paOrbital notation and subshellsOrbitals have been given names, which are usually given in the form:$${\displaystyle X\,\mathrm {type} \ }$$where X . Tingnan ang higit pa
Because of the quantum mechanical nature of the electrons around a nucleus, atomic orbitals can be uniquely defined by a set of integers known as quantum numbers. . Tingnan ang higit pa
Learn about the types, shapes, and energy levels of atomic orbitals, and how to determine their quantum numbers. Find out how to place electrons in orbitals according to Hund's .Learn about the different shapes and properties of atomic orbitals, the mathematical functions that describe the wave nature of electrons in an atom. See boundary surface . This page discusses atomic orbitals at an introductory level. It explores s and p orbitals in some detail, including their shapes and energies. d orbitals are .

Learn about the shapes, sizes and energies of s,p,d,f orbitals, the regions of space where electrons are most likely to be found. See examples, diagrams and explanations of how to arrange electrons in different .
Subshells are designated by the letters s , p , d , and f , and each letter indicates a different shape. For instance, s subshells have a single, spherical orbital, while p . An orbital is a region around an atom's nucleus where electrons are likely to be found. Different types of orbitals (s, p, d, f) have different shapes and can hold different numbers of electrons. Learn how quantum numbers are used to describe the orbitals, . An orbital is a space where a specific pair of electrons can be found. We classified the different Orbital into shells and sub shells to distinguish them more easily. This is also due to the history when they were discovered. Start with the easy. Imagine . The four chemically important types of atomic orbital correspond to values of ℓ = 0, 1, 2, and 3. Orbitals with ℓ = 0 are s orbitals and are spherically symmetrical, . An atomic orbital is the probability description of where an electron can be found. The four basic types of orbitals are designated as s, p, d, and f.Every subshell has a # of orbits s/p/d/f that can each hold 2 electrons each (one has the opposite spin of the other). The first shell (of all atoms) has 1 subshell of s-orbitals containing 1 s orbital. This means that the first shell can hold 2 electrons. The second shell has 2 subshells: 1 s-orbital and 3 p-orbitals.
The letter in the orbital name defines the subshell with a specific angular momentum quantum number l = 0 for s orbitals, 1 for p orbitals, 2 for d orbitals. Finally, there are more than one possible orbitals for l . s, p, d, and f subshells are found in the n = 4 shell of an atom. For l = 0 (the s subshell), m l can only be 0. Thus, there is .orbitals s p d f o que são orbitais Like the s and p orbitals, as n increases, the size of the d orbitals increases, but the overall shapes remain similar to those depicted in Figure \(\PageIndex{5}\). f Orbitals (l=3) Principal shells with n = 4 can have subshells with l = 3 and m l values of −3, −2, −1, 0, +1, +2, and +3.s-Orbital: sharp = kugelsymmetrisch: 0 1 p-Orbital: principal = hantelförmig: 000 3 A2: d-Orbital: diffuse = gekreuzte Doppelhantel: 0 5 f-Orbital: fundamental = rosettenförmig: 0 7 g-Orbital A1 (alphabetische Fortsetzung) = rosettenförmig: 0 9 h-Orbital A1 El número cuántico azimutal l, asociado al momento angular del electrón (cantidad de movimiento angular: el producto de su masa por su velocidad de rotación) que se expresa en letras; s para l =0; p para l =1, d para l =2, f para l =3. La nomenclatura del número l con letras tiene su origen en el estudio de los espectros de los metales .Par construction, le nombre de sous-couches par couche électronique est égal à n, tandis que le nombre d'orbitales par sous-couche électronique s, p, d, f vaut 1, 3, 5, 7, etc. Chacune de ces orbitales pouvant contenir au plus deux électrons, le nombre maximum d'électrons par type de sous-couches s, p, d, f vaut 2, 6, 10, 14. Orbital Energies and Atomic Structure. The energy of atomic orbitals increases as the principal quantum number, \(n\), increases. In any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different values of \(l\) differ so that the energy of the orbitals increases within a shell in the order .The four chemically important types of atomic orbital correspond to values of l = 0, 1, 2, and 3. Orbitals with l = 0 are s orbitals and are spherically symmetrical, with the greatest probability of finding the electron occurring at the nucleus. All orbitals with values of n > 1 and l = 0 contain one or more nodes.
orbitals s p d f|o que são orbitais
PH0 · shape of p orbital
PH1 · s p d f quimica
PH2 · orbitals chemistry
PH3 · o que são orbitais
PH4 · numero de orbitais
PH5 · niveles s p d f
PH6 · geometria dos orbitais
PH7 · atomic orbital diagram
PH8 · Iba pa